3M LifeASSURE PFS Series PTFE Filter Cartridges combine flow-enhancing construction with a liquid‐validated, bacteria retentive, hydrophobic PTFE filter membrane, providing excellent air flow rates using small assemblies. These filters are used for applications requiring 0.2 micron-sterilization of air, gas, solvents and chemicals in life science industries.
Features of 3M LifeASSURE PFS Series Filter Cartridges
- Flow-enhancing construction allows air flow with a relatively low pressure drop, resulting in a smaller vent filter assemblies
- Hydrophobic polytetrafluoroethylene (PTFE) membrane helps prevent pore blockage in vent applications, extending filter lifespan
- Liquid validation of B. diminuta retention helps provide reliable sterilizing performance in wet or dry conditions
- 100% forward flow integrity tested prior to release

|
Validated PTFE Membrane 3M LifeASSURE PFS series PTFE Filter Cartridges use a hydrophobic polytetrafluoroethylene (PTFE) membrane, which helps minimize pore blockage from moisture in vent applications. Our filters are validated by a liquid bacterial challenge for complete retention based on the ASTM standard, which provides a high level of reliable sterilizing performance. |

|
High Air Flow Construction 3M LifeASSURE PFS Series Filter Cartridges are constructed to achieve high air flow at a given pressure drop. The PTFE membrane is pleated with polypropylene nonwoven flow-enhancing layers during construction to achieve optimal flow rate performance at low differential pressures. |
|
|
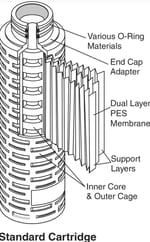
Engineered for Quality and Reliability 3M LifeASSURE PFS Series filter cartridges using all polypropylene structural components (end caps, adapters, media support, inner core and outer cage). The 3M Purification Inc. Quality Management System is approved by an accredited registering body to the ISO 9001:2008 and ISO 13485:2003 Quality Management Systems, and the cartridges are 100% forward flow integrity tested prior to shipment. All LifeASSURE PFS Series filter cartridges are shipped with a Certificate of Quality affirming compliance with manufacturing quality specifications. Supporting Drug Master File (DMF) documentation is on file with the United States Food and Drug Administration (FDA). |
| Application | Sterilizing Grade Filtration – Liquid & Gas |
|---|---|
| Brand | LifeASSURE™ |
| End Modification | 222 O-ring & Flat Cap, 222 O-ring & Spear, 226 O-ring & Flat Cap, 226 O-ring & Spear |
| Filter Material | PTFE |
| Filter Type |
Surface
|
| Formulation |
Pharmaceutical, Sterile Air, Vent Filtration
|
| Gasket | O-Ring Material |
EPR, Fluorocarbon, PTFE Encapsulated Fluorocarbon, Silicone
|
| Grade |
Sterilizing Grade
|
| Industries |
Life Sciences
|
| Length (In | Metric) | 10 in, 2.5 in, 20 in, 30 in, 5 in | 12.7 Centimeter, 25.4 Centimeter, 50.8 Centimeter, 6.4 Centimeter, 76.2 Centimeter |
| Maximum Operating Temperature (C | F) | 80° C | 176° F |
| Micron Rating | 0.2 Absolute @ 95% |
| Product Series | PFS |
| Series | LifeASSURE™ PFS Series |
| Units Per Case | 1, 6 |


